 Over a thousand papers are presented at the San Antonio Breast Cancer Symposium each year, and I attend more than 12 hours of presentations and discussions each day. It’s a lot to cover and I try to focus on the results that are most relevant and interesting to our members.
Over a thousand papers are presented at the San Antonio Breast Cancer Symposium each year, and I attend more than 12 hours of presentations and discussions each day. It’s a lot to cover and I try to focus on the results that are most relevant and interesting to our members.
I can never write up everything for the blog and anyone who is not following along on Twitter, using the identifying hashtag #SABCS19, misses much of my running commentary about the treatments and research being presented. (Note: you can follow me at @karunajaggar and Breast Cancer Action at @BCAction).
This year, I thought I’d try something new and share some of my tiny Tweets about treatment issues. For those not familiar with Twitter, it’s a platform that allows just 280 characters to make your point. While it can’t replace full presentations and analysis with nuanced discussion, it can help identify the essential points and the most important takeaways succinctly. For readers who want more, I’m including quick summaries with each Tweet below.
New Oral Chemo Option May Be on the Horizon
My Tweet: Oral administration of drugs have many advantages. New Phase III study (GS6-01) used encequidar to increase the absorption of oral paclitaxel, otherwise not absorbed orally, resulting in improved overall response rate & lower toxicity compared to IV paclitaxel. Good news 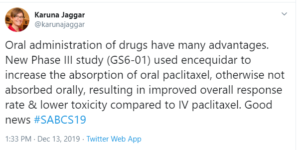
Taxanes are a foundation of breast cancer treatment but paclitaxel, which is used to treat metastatic breast cancer, is not absorbed orally. Oral administration of drugs have many advantages and a phase 3 trial presented at SABCS looked at use of encequidar to improve the absorption of oral paclitaxel. The study found that oral paclitaxel with encequidar improved response rate and overall survival, with less neuropathy, for patients with metastatic breast cancer compared with intravenous (IV) paclitaxel. It should be noted that the IV paclitaxel administration in the study was every three weeks, which is not standard dosing.
Nonetheless, this is an important study and FDA approval is being sought. This is the first orally administered paclitaxel and could have advantages. However, the tradeoff is that it involves a lot of pills: an average weight woman would have to take something like 11 pills.
Priority review for DS-8201, despite deadly toxicity
My Tweet: Data presented at #SABCS19 on trastuzumab deruxtecan (DS-8201), an experimental treatment that’s been granted a priority review by FDA in October, shows toxicity risks. It’s always a sobering reminder that cancer treatments themselves can be fatal.
Most of the excitement at SABCS in 2019 was surrounding two treatments for HER2+ metastatic breast cancer. I’ve already written about Tucatinib, which is an experimental, small molecule tyrosine kinase inhibitor that improved overall survival for people with HER2+ metastatic breast cancer, including with brain metastasis. The other experimental treatment, trastuzumab deruxtecan (DS-8201), is a novel HER2-targeted antibody–drug conjugate that has already been granted a priority review by FDA despite its toxicity risks.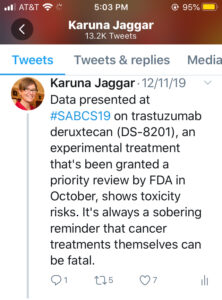
The DESTINY-Breast01 study (GS1-03) is a phase 2, open-label study that enrolled patients with HER2+ metastatic breast cancer whose tumors had progressed on T-DM1. Patients in this heavily pre-treated group had an average of six prior treatments and 13% had brain metastases. The results presented looked at a small group of 184 patients, 43% of whom were still being treated at time of analysis. The analysis is too early to have overall survival data, and the researchers reported median progression-free survival at 16.4 months.
Despite the enthusiasm, the toxicity data was sobering, and it’s always important to keep sight of the fact that cancer treatments themselves can be fatal. Fifty-seven percent of patients on trastuzumab deruxtecan (DS-8201) experienced at least one grade 3 or higher problem. The most common (13.6%) is interstitial lung disease, which caused death for four patients.
Capecitabine provides no benefit for most early stage breast cancer
My Tweet: Meta-analysis of capecitabine presented at #SABCS19 showed only small benefit overall, but the largest benefit is overall survival improvement for triple negative breast cancer treated with capecitabine in addition to other treatments.
A meta-analysis of the oral chemotherapy capecitabine, brand name Xeloda, found that adding capecitabine after surgery to standard treatment did not improve overall survival for people with early stage breast cancer. The study looked at nearly 8000 patients who received capecitabine after surgery, with a median follow up of 79 months. However, looking at a subset of patients with triple negative breast cancer, there appeared to be a small benefit in overall survival for those with residual disease after neoadjuvant chemo who added capecitabine in addition to other treatments after surgery.
De-escalation: less is best
My Tweets:
Paradigm shift: moving from maximum tolerated dose to optimal biological dose for clinical trials & drug approval. Case in point, how much tamoxifen is needed to have biological effect is a different Q than how much tamoxifen can people stomach (max tolerated dose).
#SABCS19Everyone is buzzing about “baby tam” at #SABCS19. Reduced dose Tamozifan was also hot last year: SABCS18: Happy and Hope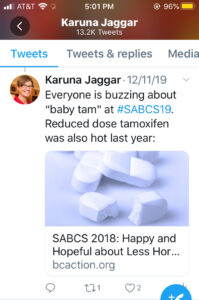 ful about Less Hormone Therapy
ful about Less Hormone Therapy
Sometimes the biggest medical advance is actually reducing treatment, so that fewer people experience the harmful effects. This could mean identifying those patients who are at lower risk who don’t need additional treatment, or identifying subgroups of patients whose cancers don’t respond to a particular treatment. Another way of de-escalating involves a fundamental paradigm shift in how dosing for breast cancer treatments are determined.
Current clinical trial design first seeks the maximum tolerated dose for new treatments and then looks at efficacy. But a paradigm shift to first seeking the optimal biological dose for clinical trials and drug approval could reduce toxicity without sacrificing efficacy. For example, asking how much tamoxifen is needed to have biological effect is a different question than how much tamoxifen people can stomach (the maximum tolerated dose). Many people with hormone positive breast cancer are excited about recent studies looking at lower dose tamoxifen, called “baby tam”, to reduce side effects without sacrificing efficacy.